|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
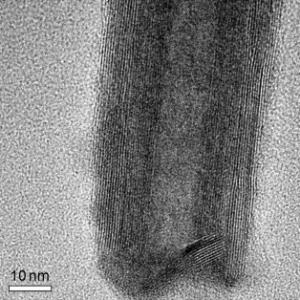
Tubes Grow From Drops |
|
Since the discovery of carbon nanotubes in the early 1990s, nanotubes and nanowires have been the focus of much scientific attention. Aside from carbon, nanotubes have since been made from various other materials. Possible applications for these nanostructures range across many fields, including microelectronic circuits, sensors, and special light conductors and light-emitting nanotubes for displays. A research team headed by Wolfgang Tremel at the University of Mainz has now developed a new process for the production of tin sulfide nanotubes. As reported in the journal Angewandte Chemie, the researchers let the SnS2 tubes “grow” out of a drop of metal. |
|
Metal sulfides with a lamellar structure that form inorganic nanotubes are not a new concept. They are currently in use in medical technology, for fibers with ultrahigh tensile strength, in hydrogen storage, for rechargeable batteries, in catalysis, and in nanotechnology. One fundamental problem with the fabrication of sulfidic nanotubes is the need for high temperatures to make the planar layers bend and fuse into tubes. In addition, they must be trapped as unstable intermediates. In the case of tin disulfide, this is nearly impossible, however, because the compound decomposes at a significantly lower temperature. The Mainz researchers thus implemented a different process for the production of tin disulfide nanotubes: they first used a vapor–liquid–solid (VLS) process, a method normally used in the production of semiconducting nanowires. Bismuth metal powder is mixed with tin sulfide nanoflakes and heated in a tube furnace under an argon stream. The reaction product is deposited at the cooler end of the tube. Nanodroplets of bismuth are formed inside the oven; these act as local points of contact for the tin. In this way, the reaction partners become concentrated within the metal droplet, which then serves as the nucleus for growth of the nanotubes. “In this process, the metal drop is obtained as a sphere at the end of the tube, and the nanotubes grow out of the sphere like a hair out of a follicle,” explains Tremel. “Catalysis by the metal droplet makes growth possible at low temperatures.” The new method allowed the scientists to produce nanotubes made of several SnS2 layers with few defects, diameters between 30 and 40 nm, and lengths between 100 and 500 nm. |
|
|
|