|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
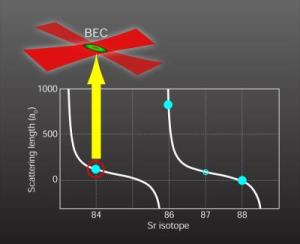
Strontium 84: |
|
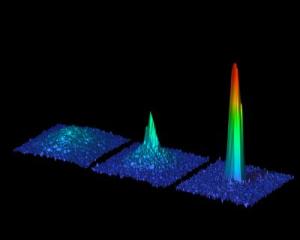
Two independent teams have, for the first time, created Bose-Einstein condensates of strontium atoms. The ability to cool strontium to very low temperatures and control its motion could lead to increasingly precise clocks and may advance our progress toward quantum computers and novel experiments in ultracold chemistry. The new Bose-Einstein condensate is reported in two papers in Physical Review Letters and highlighted with a Viewpoint in the November 9, 2009 issue of Physics (see below). When certain types of atoms are densely packed, then cooled to less than a millionth of a degree above absolute zero, they suddenly drop into a single quantum state called a Bose-Einstein condensate. In effect, the atoms combine to behave as one object rather than a group of individual pieces. This strange phase of matter was first observed in 1995 with atoms that had one electron in their outer shells. Since then researchers have achieved Bose-Einstein condensates with atoms that have two outer shell electrons, but a Bose-Einstein condensate of strontium, one of these atoms, has remained elusive. |
|
Now Simon Stellmer and colleagues at the Universit�t Innsbruck in Austria and Natali Martinez de Escobar and colleagues at Rice University in Texas have used lasers to trap and cool atoms of the isotope strontium-84 to form a Bose-Einstein condensate. If researchers can extend this achievement to other isotopes of strontium, the new Bose-Einstein condensate could improve ultra-accurate clocks, store information in quantum computers, or help test fundamental constants of nature. First Bose-Einstein condensation of strontiumQuantum physicists of Innsbruck win international race In an international first, scientists from the Institute of Quantum Optics and Quantum Information (IQOQI) produced a Bose-Einstein condensate of the alkaline-earth element strontium, thus narrowly winning an international competition between many first-rate scientific groups. Choosing the isotope 84Sr, which has received little attention so far, proved to be the right choice for the breakthrough. It can now be regarded as an ideal candidate for future experiments with atomic two-electron systems. This is not the first time that Prof. Rudolf Grimm and his scientific team have won a tightly contested race between scientists: in 2002, they were the first to produce a Bose-Einstein condensate of cesium atoms. Now junior scientist Dr. Florian Schreck and his team obtained the first Bose-Einstein condensate of strontium atoms, thus winning a tight international race despite having started their experiment much later than their competitors in the U.S.A. "We backed the right horse and, towards the end, worked day and night to realize this Bose-Einstein condensate," says Dr. Schreck. For years physicists from all over the world have tried to condense atomic strontium, focusing mainly on the two strontium isotopes that are relatively abundant in nature (86Sr, 88Sr). But the breakthrough came when Dr. Schrecks' team decided to try a new, almost counterintuitive approach. "A year ago I had the idea to try it with the isotope 84Sr which has a low natural abundance," recounts Dr. Schreck concerning the moment of breakthrough. Never considered before because of its' low natural abundance, new theoretical calculations soon showed that this neglected isotope had an ideal scattering length for producing a Bose-Einstein Condensate as compared to the other, more abundant, isotopes. Condensation of Strontium As an initial step, strontium atoms were collected in a magnetic trap and cooled using laser cooling and trapping techniques that were refined in IQOQI. In order to isolate these atoms from undesired interactions with the environment, the experiment was performed in an ultra-high vacuum chamber. After loading the atoms into an optical trap, they were cooled to near absolute zero (- 273.15 �C) by evaporative cooling techniques made possible due to the excellent scattering properties of this particular isotope � the atoms collide and thermalize without being lost due to molecule formation. This was the crucial step that was not possible with the other strontium isotopes. In this manner, a Bose-Einstein Condensate of 150,000 atoms was produced. This new form of matter is a purely quantum phenomena where the atoms lose their individual identities and coalesce into a single, collective state. The scientists of the Institute for Quantum Optics and Quantum Information (IQOQI) of the Austrian Academy of Sciences (�AW) have now succeeded in doing with strontium what other scientists have done with other chemical elements. Only two weeks after the success of the Innsbruck team, a research group from the U.S. also achieved Bose-Einstein condensation of strontium atoms. Both research results are published in the same issue of the journal Physical Review Letters. Hot Scientific Topic Strontium belongs to the atomic two-electron systems; these are elements whose atoms have two valence electrons. Most of the atoms with one valence electron have already been successfully condensed (the Nobel Prize in Physics was awarded for this achievement in 2001), making Bose-Einstein condensation of two-electron systems a new hot topic in the field of physics. Although ytterbium (2003) and calcium (June 2009) were the first two-electron systems to be condensed, it is with strontium that significantly large Bose-Einstein Condensates have been produced. With Bose-Einstein condensates research in fundamental quantum mechanics can be carried out; these include developing new schemes for quantum computation, modeling condensed matter systems, and performing precision measurements of forces on the micrometer scale, which still pose many challenges to physicists today. "The opportunities we have at the Institute for Quantum Optics and Quantum Information (IQOQI) are a crucial factor in our success," underlines Rudolf Grimm. "We had a free hand in trying something totally new and, thus, were able to enter this international race." But Dr. Schreck and his team are not stopping there. They have already started working on a new exciting direction; besides the three bosonic isotopes of strontium mentioned above, there is a fermionic isotope, 87Sr, which Dr. Schreck intends to use to produce the first ultracold Fermi gas of strontium atoms. Rice University: Rice ties in race for atomic-scale breakthroughKillian lab creates Bose-Einstein condensate from strontium. Everybody loves a race to the wire, even when the result is a tie. The great irony is the ultraprecise clocks that could result from this competition could probably break any tie. The Rice lab of physicist Tom Killian published a paper online this month demonstrating the long-sought creation of a Bose-Einstein condensate (BEC) of strontium atoms. BEC is a state of matter predicted by early 20th-century physicists Satyendra Nath Bose and Albert Einstein. Basically, at extremely cold temperatures, some types of atoms come to almost a complete standstill and enter a state in which they lose their individual identity. In the same online edition of Physical Review Letters, a paper by the laboratory of Rudolf Grimm at the Universit�t Innsbruck in Austria reported the same result. Though it was a scramble to the finish line, in the end a gentlemanly agreement determined the knotty outcome. Killian said he caught wind of the Austrians' work in an online archive where physicists commonly post preprints of upcoming papers. "We've both been working on this for a long time, but when their paper appeared on the archive, there was still a technical problem keeping us from getting a BEC," said Killian, an associate professor of physics and astronomy. "After three or four all-nighters, we fixed our problem, wrote the paper and submitted it. At that point I discovered the Austrian paper had been fast-tracked and publication was imminent." Killian got on the phone with the journal's editor. "He called Austria, and they said they'd be happy to wait for us. They were very generous." Killian said his lab had been working toward a BEC for years and avoiding trial and error in favor of measurements to determine which isotope of strontium was most likely to condense. To get the atoms to stop in their tracks and form a BEC, the lab had to cool them to near absolute zero (-459.67 degrees Fahrenheit) through a combination of tried-and-true techniques involving lasers and evaporative cooling. That brings the trapped atoms, which are inside an evacuated chamber, to within a millionth of a degree of zero Kelvin. At that point, the atoms collapse into a condensate -- a singular lump that physicists can play with. Physicists have been making BECs since 1995, when Eric Cornell, Carl Wieman and Wolfgang Ketterle confirmed Bose and Einstein's theory with ultracold atoms and won the Nobel Prize for their efforts. Randy Hulet of the Rice Physics and Astronomy Department has also made seminal contributions to the field from its beginning. A number of elements have since been used to make BECs, most of them alkali-metal atoms, which have one valence electron. The number of valence electrons, which spin in the outer shell of an atom, determines how it reacts with other atoms. Strontium has two valence electrons, which really changes the game. "In the lowest energy state they spin in opposite directions and pair up very nicely," said Killian. Applying just the right amount of energy via a laser can flip the spin of one of the electrons. Once they're aligned, in the case of strontium, they stay that way for minutes. "That long-lived, excited state allows you to lock your laser to just the right energy for spin flipping," he said. "This controls the frequency of the laser, or the rate at which the waves go by. Those waves become the pendulum of the world's most accurate clock." Killian expects the combination of clock technology and BECs can lead to breakthroughs in quantum computing. In 2004, a BEC in ytterbium, another two-valence-electron atom, was observed; but strontium is used in the best clocks, and strontium BECs contain 10 times more atoms, which is important for future experiments. Killian expects the Austrian lab will pursue quantum computation, but his interests lie elsewhere. "It turns out that, for the same reasons these atoms make a good clock, we can use lasers to manipulate the way the atoms interact with each other. "Lasers can change interactions on small length scales and very quickly, which opens the study of fundamental phenomena in solid-state physics and materials we don't quite understand," he said. Killian said manipulating interactions could also lead, someday, to matter-wave "lasers" for very precise sensors. "That has applications for things like navigating airplanes or submarines, detecting oil deposits underground or tunnels where tunnels shouldn't be, like along international borders," he said. "I do really fundamental physics, but if we work on these techniques, 10 to 15 years from now they could have applications," Killian said. "The specific properties of strontium make it very useful for some of these applications, which is why it's valuable." |
|
|
|