|
|
|
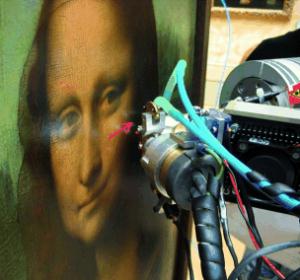
Not just a pretty face: Non-invasive X-ray fluorescence spectroscopy was used to reveal the sfumato paint layer stacking method that was used by Leonardo da Vinci to paint the faces in seven of his paintings. A strong diversity in his technique could clearly be seen with this method.
[Credit: Angewandte Chemie]
|
Da Vinci’s technique is fascinating. The gradation of color from light to dark is barely perceptible and looks natural. “Neither brushstroke nor contour is visible: lights and shades are blended in the manner of smoke,” says Walter. The details of how the sfumato technique worked have not been determined before. Walter and his colleagues have now used a non-destructive technique, X-ray fluorescence spectroscopy, to track down the secret. The paintings were irradiated with X-rays. Every chemical element then gives off a characteristic fluorescent light, which allows the element to be quantified. “Until now, the analysis had remained qualitative, because all the pigment layers were considered simultaneously,” reports Walter. “New technical advances and software have now allowed us to resolve cross-sections of the layers and to quantitatively analyze the composition and thickness of the individual pigment layers.” The seven paintings examined - including the Mona Lisa - span over 40 years of Da Vinci’s work. In the Mona Lisa, the darker areas arose because a manganese-containing layer was applied more thickly than in the lighter areas. The underlying layers containing lead white are equally thick all over. In a painting dating from about ten years earlier, “Belle Ferronnière”, things are different: Here the shade effects are not the result of a glaze shining through; instead, Da Vinci seems to have used a covering layer of color - dark pigments in a classic oil technique,” says Walter. “The master continuously improved his painting technique. In his later paintings he was then able to produce translucent layers made of films of an organic medium ranging from 30 to only a few micrometers in thickness - an amazing achievement even by today's standards.” The long drying time of the individual layers, lasting weeks and months, explains why Da Vinci worked on the Mona Lisa for over four years, leaving the painting unfinished, according to texts from the Renaissance period.
|