|
Related Topics: |
|
Current Research Articles: |
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Findings a step toward making new optical materials |
|
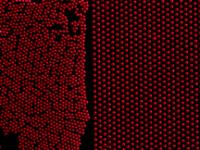
The method, developed at Purdue University, works by positioning tiny particles onto a silicon template containing precisely spaced holes that are about one-hundredth the width of a human hair. The template is immersed in water on top of which particles are floating, and the particles automatically fill in the holes as the template is lifted. |
|
The researchers have used the technique to create a "nearly perfect two-dimensional colloidal crystal," or a precisely ordered layer of particles. This is a critical step toward growing three-dimensional crystals for use in optical technologies, said You-Yeon Won, an assistant professor of chemical engineering. "Making the first layer is very difficult, so we have taken an important step in the right direction," Won said. "Creating three-dimensional structures poses a big challenge, but I think it's feasible." Findings were detailed in a paper appearing online April 9, 2008, in the journal Soft Matter, published by the Royal Society of Chemistry in the United Kingdom. The paper was written by graduate student Jaehyun Hur and Won. The single-layer structures might be used to form "micro lenses" to improve the performance of optical equipment, such as cameras and scientific instruments, and to control the color and other optical properties of materials for consumer products. More importantly, the technique represents one of several possible approaches to create "omni-directional photonic band gap materials." Unlike conventional mirrored materials, which reflect light hitting the mirror at certain angles, the omni-directional materials would be "perfect mirrors," reflecting certain wavelengths of light coming from all directions. The materials would dramatically improve the performance of optical fibers, which contain a mirrored coating to keep light from escaping. Omni-directional coatings would increase how much light is transmitted by fiber-optics and could possibly be used in future sensor technology and "optical computers" and circuits that use light instead of electronic signals to process information. It might be possible to use Won's method to create special crystals with particles arranged in the same pattern as carbon atoms in diamonds. The first layer could be a starting point for growing the crystals. "There is no conventional technology that allows you to easily fabricate the diamond-crystal structure, so our method could open the door to doing so," Won said. However, other researches are trying to use competing technologies to create photonic bandgap materials made not of the crystals but of different structures. One such competing concept is using photolithography, the same technology used in computer-chip manufacturing, to create structures resembling log piles made of tiny rods. Won's approach to precisely manipulate small particles suspended on the surface of water is difficult because inherent thermal energy causes the particles to constantly vibrate, a phenomenon called Brownian motion. To produce the single-layer structure, the engineers used a process called Langmuir-Blodgett monolayer deposition, a standard technique used in physical chemistry, primarily to create lipid membranes for research. "Using the Langmuir-Blodgett process offers a small window of processing opportunity to manipulate these small-size particles without getting too much interference from Brownian motion," Won said. "The key discovery here is the process. We've demonstrated a new process, and we discuss the science behind this process." Self-assembly is potentially promising for future manufacturing because devices could be made less expensively than using conventional processes, which require complex etching and other techniques common in the semiconductor industry. The method developed at Purdue is faster and would be far less expensive than a competing method for creating the crystals, a technique called "nano-robotics," in which particles are individually placed in a template using a robotic arm. "We envision that this self-assembly method will open a new possibility for mass fabricating complicated 3-D colloid crystal structures for various applications," Won said. The Purdue-developed technique takes about 20 minutes to create a structure that would take weeks to produce using nano-robotics. The single layer of particles forms at the surface of water in a trough-like vessel. As the template is pulled vertically out of the trough, the particles are pushed into the template holes by capillary force, the same phenomenon that causes water to rise to a higher level in a tube placed in a pool of water. It's critical for the particles to be spaced properly prior to the Langmuir-Blodgett deposition so that water can draw the particles into the holes in the template using capillary force, Won said. Researchers found that it was essential to control three conditions to successfully create the layer of particles: humidity, how fast the template is lifted out of the solution and the initial density of particles in the solution. The researchers discovered that defects form when the air is too dry. "When we suppressed water evaporation by humidifying the area, we created a completely flat, horizontally uniform structure," Won said. "Water evaporation causes a non-uniform structure formation on the surface. This is a huge problem because our goal is to make two-dimensional crystal structures as uniform as possible over the entire patterned region. By controlling humidity, we proved that we can solve that problem." It was the first time researchers had demonstrated how to create a uniform structure over the relatively large area of such a templated region, which measured about 9 square millimeters, or large enough to contain about 1.7 million particles. The engineers were able to precisely control the particle density, or how many particles occupy a given space, by using two Teflon bars like bookends on either end of the particle layer formed on the water surface to compress the particles before being deposited. The particles in the research had a diameter of about one micron, or millionth of a meter. Producing a high-quality single layer of micron-size particles has proven difficult for researchers until the new technique was developed. "That's because it is very difficult to manipulate those small particles to make a well-aligned, well-arranged structure," Won said. The researchers used their technique to make layers in various patterns, such as square or hexagonal. "We can make whatever structure we want," he said. Other researches have created self-assembling layers of particles without controlling the spacing between particles, resulting in "close-packed structures," which cannot be used to build three-dimensional, high-quality photonic crystals. Using a template enabled the researchers to create the precisely controlled pattern of particle spacing, a "non-close-packed" first layer, which is critical to building up to a three-dimensional crystal with an arbitrary, desired optical property. The researchers used an optical microscope and imaging-analysis techniques to count the number of particles in the layers they created. The engineers also created a theoretical model that describes how altering the three conditions of particle density, humidity and template lifting speed affect the quality of the structures. The model has been used to determine the exact experimental conditions needed for creating the perfect crystal structure. The particles were made of silica attached to a chemical group called hydroxyl, which is made of an oxygen and hydrogen atoms. The Purdue researches currently are investigating how difficult it would be to create three-dimensional crystals from the two-dimensional structures. Omni-directional materials currently are prohibitively expensive to manufacture. Developing an affordable manufacturing technique would be a breakthrough, Won said. The research has been funded in part through the Purdue Research Foundation and the American Chemical Society's Petroleum Research Fund. |
|
|
|
|
Related topics - search form: |
|
|