|
Related Topics: |
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Technological breakthrough in the fight to cut greenhouse gases |
|
The Newcastle University team, led by Michael North, Professor of Organic Chemistry, has developed a highly energy-efficient method of converting waste carbon dioxide (CO2) into chemical compounds known as cyclic carbonates. The team estimates that the technology has the potential to use up to 48 million tonnes of waste CO2 per year, reducing the UK's emissions by about four per cent. |
|
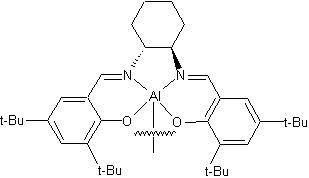
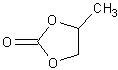
Cyclic carbonates are widely used in the manufacture of products including solvents, paint-strippers, biodegradable packaging, as well as having applications in the chemical industry. Cyclic carbonates also have potential for use in the manufacture of a new class of efficient anti-knocking agents in petrol. Anti-knocking agents make petrol burn better, increasing fuel efficiency and reducing CO2 emissions. The conversion technique relies upon the use of a catalyst to force a chemical reaction between CO2 and an epoxide, converting waste CO2 into this cyclic carbonate, a chemical for which there is significant commercial demand. The reaction between CO2 and epoxides is well known, but one which, until now, required a lot of energy, needing high temperatures and high pressures to work successfully. The current process also requires the use of ultra-pure CO2, which is costly to produce. The Newcastle team has succeeded in developing an exceptionally active catalyst, derived from aluminium, which can drive the reaction necessary to turn waste carbon dioxide into cyclic carbonates at room temperature and atmospheric pressure, vastly reducing the energy input required. Professor North said: 'One of the main scientific challenges facing the human race in the 21st century is controlling global warming that results from increasing levels of carbon dioxide in the atmosphere. 'One solution to this problem, currently being given serious consideration, is carbon capture and storage, which involves concentrating and compressing CO2 and then storing it,' he said. 'However, long-term storage remains to be demonstrated'. To date, alternative solutions for converting CO2 emissions into a useful product has required a process so energy intensive that they generate more CO2 than they consume. Professor North compares the process developed by his team to that of a catalytic converter fitted to a car. 'If our catalyst could be employed at the source of high-concentration CO2 production, for example in the exhaust stream of a fossil-fuel power station, we could take out the carbon dioxide, turn it into a commercially-valuable product and at the same time eliminate the need to store waste CO2', he said. Professor North believes that, once it is fully developed, the technology has the potential to utilise a significant amount of the UK's CO2 emissions every year. 'To satisfy the current market for cyclic carbonates, we estimate that our technology could use up to 18 million tonnes of waste CO2 per year, and a further 30 million tonnes if it is used as an anti-knocking agent. 'Using 48 million tonnes of waste CO2 would account for about four per cent (based on 2004 figures from the UN. Source: Wikipedia) of the UK's CO2 emissions, which is a pretty good contribution from one technology,' commented Professor North. The technique has been proven to work successfully in the lab. Professor North and his team are currently carrying out further lab-based work to optimise the efficiency of the technology, following which they plan to scale-up to a pilot plant. |
|
|
|
|
Related topics - search form: |
|
|