|
Related Topics: |
|
Current Research Articles: |
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Researchers reveal structure of protein that repairs damage to cancer cells |
|
One protein detects and repairs damage in malignant cells that may result from a certain type of cancer therapy. In a paper published in the April 24 issue of the journal Nature, the team raised the possibility of designing a molecule that could interfere with the repair process, making cancer treatment more effective. Chuan He, Assistant Professor in Chemistry, led the team. His co-authors included Phoebe Rice, Associate Professor in Biochemistry and Molecular Biology at the University of Chicago, and five researchers from his laboratory: Cai-Guang Yang, Chengqi Yi, Erica Duguid, Christopher Sullivan, and Xing Jian. Their work was supported by the National Institutes of Health, the W.M. Keck Foundation and the Arnold and Mabel Beckman Foundation. |
|
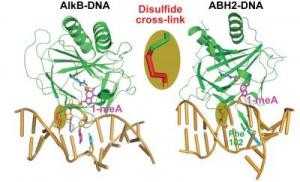
In their Nature paper, the scientists determined, for the first time, the crystal structures (showing the three-dimensional framework of atoms) of two related DNA-repair proteins bound to double-stranded DNA: a bacterial protein called AlkB, and a corresponding human protein, ABH2. Scientists had been seeking the structures of these proteins to better understand how they perform their key roles in repairing DNA. The bacterial protein can bind to either single- or double-stranded DNA. The strands separate during the replication process, but the bacterial protein avoids the latter. “This is very bizarre, because most other DNA repair proteins prefer double-stranded,” He said. The AlkB protein avoids double-stranded DNA because binding it takes more energy. “Double-stranded DNA is rigid. Single-stranded is very flexible,” He explained. “You can cause all kinds of distortion in single-stranded without paying much energetic penalty.” Many labs have unsuccessfully attempted to solve the structure of the bacterial protein with double-stranded DNA. They failed because this family of proteins binds DNA weakly, which foils the application of traditional crystallographic methods, He said. His team reinforced the complex by cross-linking the DNA to the protein. Previous crystal structures of this protein included only a very short, single-stranded segment of DNA, and did not reveal all of its interactions with larger, more biologically relevant strands of DNA. “The technique that they used to grow these crystals is very clever,” said Rice, who served as the team’s crystallographer. “It’s a nice application of chemistry to solving important questions in biology. “Knowing which pieces of the enzyme are important for interacting with double-stranded DNA and which pieces are missing when it would prefer a single strand will help us to predict the functions of related proteins,” Rice said. Said He: “We’re now applying this same strategy to all kinds of other protein-DNA complexes.” The AlkB and ABH2 proteins repair alkylation damage to DNA, including the damage caused by alkylating cancer treatments. Alkylation is the addition of certain chemical groups to DNA and is particularly harmful to rapidly growing cells such as cancerous ones. In an unexpected development last year, a team of European researchers published studies that identified an obesity gene in humans that belongs to the same family of proteins as AlkB. A defect in this gene, FTO, is associated with a weight gain of nearly seven pounds. “It’s surprising and exciting to see that the demethylation function is linked to obesity,” He said. “Methylation is a type of alkylation that is used to regulate gene expression.” One of his future goals is to determine the function of the FTO protein. Among their many functions, proteins control chemical reactions in the cell by turning genes off and on. “This family of proteins might use demethylation as a signal to regulate gene activation,” he said. “But this type of demethylation by AlkB and FTO has never been linked to gene activation in the past.” He oversees a research team of approximately 20 students and postdoctoral scientists, which occupies nearly half of one wing on the third floor of the Gordon Center for Integrative Science. The team collects most of its data at the Department of Energy’s Advanced Photon Source at Argonne National Laboratory. Over the course of many data-collection visits to Argonne, He’s team has become well-acquainted with the scientists assigned to the lab’s Structural Biology Center and BioCARS (Center for Advanced Radiation Sources). “After they learned of this work, they provided some new ideas that we’re collaborating on,” He said. |
|
|
|
|
Related topics - search form: |
|
|