|
Related Topics: |
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Nano sculptures in gold |
|
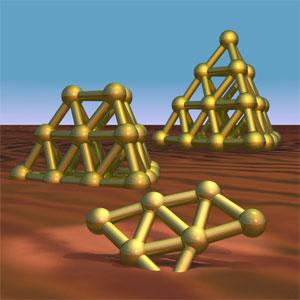
If someone is charged up, the colour of their face might change, but they don’t immediately pull off one of their arms, only to reattach it as a third leg. With some molecules, however, the situation is quite different - for example, in a gold cluster with seven atoms. In a charged state, the atoms arrange themselves differently than when they are uncharged. This was discovered by scientists at the Fritz Haber Institute of the Max Planck Society in Berlin, the Steacie Institute for Molecular Sciences in Canada and by scientists working with the FELIX free electron laser at the FOM Institute in Rijnhuizen in the Netherlands. The researchers ingeniously combined an infrared spectrometer and a mass spectrometer to show the structures of uncharged gold nano particles for the first time. These particles are currently being considered as catalysts that support certain chemical reactions. |
|
Chemically inert and expensive - these characteristics have dampened chemists’ enthusiasm for gold - at least since the era of alchemy came to an end. However in recent years interest in the element has been rekindled. "It is possible that nano particles of precious metals are suitable candidates for catalysts in important reactions in the chemical industry," says André Fielicke, who headed up the work carried out by the Berlin-based researchers. This is because these tiny gold particles are very selective about which reactions they assist in. Whether the gold nano particles favour certain reactions depends very much on their structure. Scientists at the Fritz Haber Institute in Berlin have now developed a method to determine the structure of neutral gold clusters. Chemists have known for some time what some of these collections of up to a few dozen charged atoms look like. However previously it was uncharged particles that would have attracted interest as catalysts - and they sometimes take on a quite different form from those of charged clusters with the same number of atoms. The Berlin researchers examined clusters with 7, 19 and 20 atoms. In the uncharged clusters with 19 and 20 atoms they observed the same structures that are familiar from their negative counterparts: 20 gold atoms stacked into a tetrahedron, a pyramid with a triangular base. With one atom fewer, the pyramid loses its tip. "Seven uncharged gold atoms form a triangle with an additional vertex," says André Fielicke. In a positively charged cluster, on the other hand, seven atoms form a hexagon with one atom in its centre. In uncharged form, there are three gold atoms positioned on each edge of the triangle. On one edge, two atoms are bridged by another, which creates the extra vertex. "Uncharged gold atoms probably prefer this structure as it is easier for the electrons to avoid each other," says Fielicke. In order to scan the structure of the uncharged nano particles, the scientists in Berlin had to solve several problems at once. "The clusters are rather unstable; you can’t just buy them as a powder," explains Philipp Gruene, who carried out a large portion of the experiment. Therefore, the scientists had to create the gold clusters in the same apparatus in which they determine the particle structure. To this end, they use a laser to vaporize small quantities of the precious metal from a gold bar that creates gold clusters in different shapes and sizes. In this confusion of particles, it is not possible to discern any particular structures, which poses the next problem. Normally, chemists separate a mixture of particles like this in a mass spectrometer. This device first ionizes the particles, which means it provides them with an electrical charge. Then it separates them according to their mass in an electrical field, as the field accelerates lighter particles faster then heavier ones - when they both carry the same charge. If there is a large quantity of one kind of particle, its structure can be resolved in an infrared spectrometer. In such an apparatus, infrared light makes the particles vibrate in different ways depending on their wave length, that is, the colour of the light. Many particles can be identified on the basis of the different vibrations they are able to generate. This is because the options for vibration are a factor of the molecule’s shape. For example, a ring-shaped molecule pulses quite differently than a molecule with a long shape, even if it contains the same atoms. The vibration in question is revealed by the wavelength that registers on the scale of the infrared spectrometer. Since the scientists in Berlin want to observe the structures of uncharged particles and can only produce very few of them, this procedure is excluded. Nevertheless, they use both methods, but combine them in a very ingenious way. Before they separate out the confusion of particles, they fire a very intense infrared laser with a certain wavelength at the mix. The laser light is so intense that it brutally separates the clusters. The intense light makes some of the particles vibrate so violently that they burst. After selection with the infrared laser, the researchers send the mix of the particles that remain through a mass spectrometer. The particles that have been excited and destroyed by the infrared light of a certain wavelength leave hardly any traces in the mass spectrum. The scientists use a control experiment to establish that there is something missing from a certain point in the mass spectrum: they also separate a mix of gold clusters in the mass spectrometer, which they previously have not subjected to special treatment with the intensive infrared laser. Conducting an experiment like this with a laser beam that has a single wave length is not very productive. Only a full vibration spectrum reveals the structure of a particle. "This means we have to repeat the experiment with around 200 different wavelengths of the infrared laser," says André Fielicke. That creates the next problem for the scientists: only a free electron laser delivers laser light that is sufficiently intense over most of the spectrum to make the clusters burst. The Berlin-based researchers therefore resolved the structure of the gold clusters on the Free Electron Laser for Infrared eXperiments - Felix - in Nieuwegein in the Netherlands. They reconstructed the vibration spectrum for certain clusters from the mass spectrometer readings at different wave lengths of the infrared laser, allowing them to see their structures. With their investigations, the scientists in Berlin are helping in the search for a catalyst for epoxidization - a technically important reaction in which chemists attach hydrocarbon molecules to an oxygen atom. This is often the first step towards creating more complex molecules. Whether the end result yields the desired product depends on where the oxygen atom attaches itself to the hydrocarbon. And that’s where the gold clusters come in - they could act as pilots. |
|
|
|
|
Related topics - search form: |
|
|