|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
All the Colors of the Rainbow |
|
Butterfly wings are colorful even though they contain no pigments. Their shimmering color stems from wavelength-dependent light scattering by the nanostructured surfaces of their wings. A similar effect can be achieved with photonic crystals. A Canadian and British research team has now developed a highly effective photonic crystal whose color can be adjusted continuously from UV through the wavelengths of visible light and into the near-infrared range. As they report in the journal Angewandte Chemie, this material could be useful for the production of reflective full-color displays in applications such as electronic books. |
|
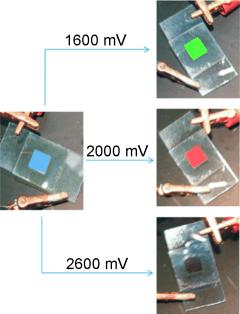
Photonic crystals for full-color displays represent a special technical challenge. They must be able to change their optical properties in response to an external stimulus such as electrical voltage; they must do this continuously, reversibly, and very rapidly, and must be obtainable in large quantities at low cost. A team led by Geoffrey A. Ozin at the University of Toronto (Canada) and Ian Manners at the University of Bristol (UK) has now successfully developed such a material. It is a polymer gel with an inverse opal structure. An opal is a colorfully shimmering gemstone made of nanoscopic, densely packed silicate spheres that scatter incident light. The researchers have produced an inverse opal structure by depositing a thin layer of silicate spheres onto a substrate and filling the unoccupied spaces with an electrically conducting polymer, which they then cross-linked. By etching away the silicate, they produced a highly porous polymer gel with a lattice-like structure. Where an opal structure would have its spheres, this material contains cavities, which the researchers filled with an electrolyte solution. If a voltage is applied, electrons are drawn out of the polymer. In order to achieve charge neutrality, negatively charged anions from the electrolyte and solvent diffuse into the polymer gel causing the gel to swell. If the voltage is reduced, the electrons are pushed back into the polymer and the anions and solvent are expelled, causing the gel to shrink. The large interior surface that comes into contact with the electrolyte makes these processes extremely fast. Changes in the dimensions of the lattice change the wavelength of the light reflected by the material. Depending on the voltage, the lattice dimensions can be adjusted seamlessly, so that the entire color spectrum of visible light can be covered. For example, at 2 V the gel looks red, while at 1.6 V, it appears green. |
|
|
|