|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
An old dream has been fulfilled: |
|
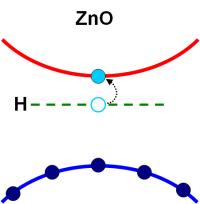
Zinc oxide is a "jack of all trades" - thousands of tons thereof are produced all over the world every year. Its fields of utilization range from food additive to sun screening agents. It is even significant as semiconductor, although the great breakthrough in this field is yet to come. Perfect doping is not yet possible. A team of chemical scientists at the Ruhr-University in Bochum, working under the auspices of Prof. Christof Wöll, is a step closer to unveiling the reason. They were experimentally able to provide evidence that hydrogen atoms disturb the process. |
|
Controlled concentration of hydrogen atoms during the production of intrinsic zinc oxide is thus the key to the routine use of ZnO as semiconductor. The scientists have documented their results in the journal Physical Review Letters. Doping activates the semiconductor Doping, i.e. the insertion of specific foreign atoms into the crystal lattice of a solid, is the most important factor during the production of semiconductor devices. These foreign atoms either release an electron (n-doping), or absorb an electron, thus creating a "hole" in the solid (p-doping). These mobile electrons or holes then bring about the electric conductivity of the otherwise isolated semiconductor, i.e. doping initiates "action" in the semiconductor. This standard process in the manufacturing of conventional semiconductors e.g. silicon or germanium has however been problematic for zinc oxide to date. In particular, it has been difficult to achieve p-doping, which made it impossible to construct semiconductor devices such as transistors or light emitting diodes (LEDs). Such devices require a pn-transition, a junction between the p-doped and the n-doped zones. Thus, in the field of semiconductors, zinc oxide is at present only used for a few special applications. Hydrogen is always present There has been a significant improvement in the production of intrinsic zinc oxide during the past few years. Blue LEDs made of zinc oxide have only been presented recently. There are, however, still numerous problems concerning doping. Research scientists at the Ruhr-University in Bochum have been able to identify a significant obstacle in the production of intrinsic zinc oxide. During experiments, which had actually been motivated by an interest in the catalytic properties of ZnO, they were able to show that hydrogen atoms always result in n-doping. They could reversibly dope zinc oxide substrates using hydrogen and then eliminate the hydrogen by heating. The scientists were thus able to verify theoretical predictions made in 2000. They used a special technique for measurements at diverse temperatures to verify the corresponding charge carrier concentrations. A sufficiently high density of these charge carriers is essential for the proper functioning of electronic devices. Hydrogen impurities are almost impossible to avoid during the production process, thus preventing the targeted p-doping. The electrons released from the H atoms to the ZnO immediately fill the holes caused by the p-doping. High purity, in particular a hydrogen-free environment, is thus a decisive factor for the production of intrinsic zinc oxide. Controversy is history The research team was thus also able to resolve a scientific controversy: to date it has often been postulated that the doping problems are caused by imperfections in the zinc oxide crystal lattice, by additional Zn atoms or oxygen defects. The results obtained in Bochum are a basis for the production of higher performance ZnO-based electronic circuits. Currently the scientists are doing intensive research to attain p-doping with an intrinsic, i.e. hydrogen-free, zinc oxide substrate by incorporating appropriate foreign atoms. Technology actually intended for another purpose The technology used, a special version of electron spectroscopy, is normally used for another purpose, namely for the investigation of chemical processes on the surfaces of zinc oxides. Such phenomena have been investigated at the Ruhr-University in Bochum for many years within the frameworks of SFB 558 (Focus Research Centre - Metal-Substrate Interactions in Heterogeneous Catalysis) due to the significance of zinc oxide for heterogeneous catalysis, particularly for the synthesis of methanol. |
|
|
|