|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Kilogram Quantities At Last! |
|
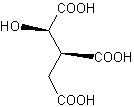
The citric acid cycle, one of the most important metabolic processes in our bodies, was formulated in 1937. Since then, all of the intermediates have been produced in multigram quantities - with one exception, (2R,3S)-isocitric acid. Athanassios Giannis and his team at the University of Leipzig have finally done it. As they report in the journal Angewandte Chemie, their process, a combination of one biotechnological and one chemical step, starts with sunflower oil, a renewable starting material. Isocitric acid and its derivatives thus become accessible on a kilogram scale. |
|
In the citric acid cycle, acetyl CoA, formed in the breakdown of lipids, sugars, and amino acids, is used to produce energy that is biochemically available to an organism. Carbon dioxide and water are produced in this process. This reaction mechanism is named after one of the intermediate products, the anion of citric acid. In nature, isocitric acid is always found with its isomer, citric acid. The difference between these two compounds is merely that the hydroxy group (-OH) is bound to a different carbon atom of each molecule. Large-scale separation of the two isomers has not been possible. A fermentative synthesis of the pure compound has also not worked. Giannis team has now finally done it, thanks to a host of tiny helpers, the yeast Yarrowia lipolytica, which produces isocitrate from refined sunflower oil in previously unachievable yield and in a favorable isocitrate to citrate ratio. After the biomass is filtered out, electrodialysis is used to obtain the pure acids. The researchers use a trick to separate citric acid from isocitric acid: They use methanol to convert the compounds into the corresponding methyl esters. Why does this work? Whereas the citric acid ester crystallizes, the isocitric acid ester is a liquid. Separation then becomes child�s play. Why was isocitric acid so important to these researchers? Isocitric acid is a compound with chiral centers - carbon atoms with four different groups bound to them. There are always two versions of a chiral center, one being the mirror image of the other. Smaller, easily accessible chiral compounds are useful building blocks for the synthesis of complex natural products and are interesting starting materials for the pharmaceutical industry. Isocitric acid makes available a new assortment of such chiral building blocks. |
|
|
|
|
Related topics - search form: |
|
|