|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
'Hot' oxygen atoms on titanium dioxide motivated by more than just temperature |
|
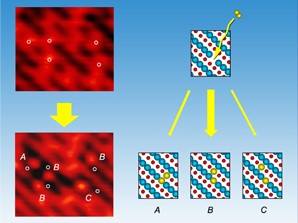
RICHLAND, Wash. � Like two ballroom dancers waltzing together, the two atoms of an oxygen molecule severed by a metal catalyst usually behave identically. But new research reveals that on a particular catalyst, split oxygen atoms act like a couple dancing the tango: one oxygen atom plants itself while the other shimmies away, probably with energy partially stolen from the stationary one. Scientists from the Department of Energy's Pacific Northwest National Laboratory found the unanticipated behavior while studying how oxygen interacts with reduced titanium oxide surfaces. The chemists are trying to understand how molecular oxygen - the stuff we breathe - interacts with metals and metal oxides, which are used as catalysts in a variety of environmental and energy applications. Researchers worldwide are exploring the use of titanium dioxide, especially in hydrogen production for solar fuel cells. |
|
The team was a bit surprised by the unequal sharing of resources among the oxygen atoms. "It is unique that one atom stays in place and the other one is mobile and probably gets most of the energy," says lead scientist Igor Lyubinetsky, who performed the work at the The Environmental Molecular Sciences Laboratory, a DOE national scientific user facility located at PNNL, with funding by DOE's Office of Basic Energy Sciences. Their work will be published as the cover article in the Journal of Physical Chemistry C on February 21, 2008, and previously appeared online January 5, 2008. Researchers have yet to determine if this short-lived extra mobility plays a role in chemical reactions, but understanding the basic chemistry might be important in processes that break down pollutants or split water to generate hydrogen. Previous research has revealed much about how oxygen molecules interact with metals. For example, when molecular oxygen (O2) hits a platinum surface, the platinum helps split the molecule apart and each oxygen atom zips over the surface in opposite directions, eventually sticking to the metal. Chemists call the pumped up atoms "hot" because the extra energy released by the breaking and reforming bonds gives the atoms their boost. Titanium dioxide is not only a popular catalyst, but it also serves as a great model oxide to study basic chemistry. PNNL scientists, led by Lyubinetsky, wanted to know if molecular oxygen behaved on titanium dioxide the way it behaves on metals such as platinum. Oxides have different properties than metals: Rust, for example, is iron oxide, which flakes off from iron metal. To find out, the team started with a slice of titanium oxide crystal, oriented so that titanium and oxygen atoms line up on the surface in alternating strips, forming grooves of titanium troughs between oxygen rows. By heating the sample, the team created imperfections on the surface, or spots where an oxygen atom vacated its row. Using scanning tunneling microscopy, the researchers found that molecular oxygen only broke apart when it encountered a vacancy, indicating that oxygen molecules bounce along flawless titanium oxide surfaces and don't react, as expected from previous results. The team also expected one of the atoms to make the vacancy its home, and the second to situate itself right next to its former partner. Instead, the chemists found that the second oxygen behaved like a "hot" atom and was free to move one or two crystal lattice spaces away. Out of 110 molecules the team counted, more than three quarters of the hot atoms hopped one or two spaces away before becoming mired on the surface. "This is one of the first time chemists have looked at oxygen on metal oxides at the atomic level, and this finding was unexpected," says Lyubinetsky. But a skittering atom requires some sort of energy to propel it, so the researchers explored how a splitting oxygen molecule divvied up its energetic resources. The team found that a free oxygen atom at room temperature (about 20 C or 68 F) is virtually immobile on a titanium oxide surface. However, previous calculations have suggested that the energy is released from the rearrangement of the bonds -- from within the oxygen molecule and between the oxygen atom and titanium surface - and the team has concluded this might be the source of the hot atom's burst after its partner anchored itself in the vacancy: the calculated energy was about two to three times that required to get an immobilized oxygen unstuck. Lyubinetsky postulates that the hot oxygen atom uses this energy to move around on the titanium oxide surface. The scientists are trying to better understand the mechanism because it might be significant in basic catalytic chemistry. "This finding may be important in surface reactivity. We don't know yet," Lyubinetsky says. The chemical event could, for example, be affected by the extra energy the oxygen atom possesses. The effect might also play into whether surface oxygen atoms interfere with the chemistry between the catalyst and other reagents. In any event, the result will keep chemists tango-ing with new questions for a long time. |
|
|
|
|
Related topics - search form: |
|
|