|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Chemists measure copper levels in zinc oxide nanowires |
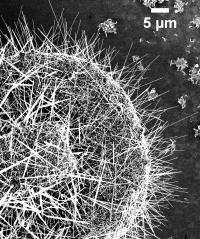
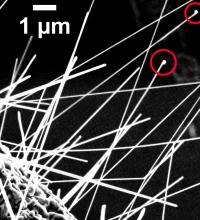
Chemists at the National Institute of Standards and Technology (NIST) have been the first to measure significant amounts of copper incorporated into zinc oxide (ZnO) nanowires during fabrication. The issue is important because copper plays a significant - but not well-understood - role in important optical and electrical properties of the nanowires. Previous experiments found only trace amounts of copper. Although zinc oxide is best known as a strong sunblock, cold remedy, itch reliever and paint pigment, nanotech engineers like it for its photoluminescence (the ability to emit light after absorbing electromagnetic radiation), field emission (the basis for advanced, high-definition flat-panel displays) and piezoelectric properties (stressing or changing shape when electricity is applied and producing electricity when stressed). ZnO nanomaterials may one day be used to improve solar cells, lasers, sensors, ultraviolet light sources, field emission sources and piezoelectric devices. Copper enters the ZnO nanowires during fabrication. The nanowires - about 50 to 150 nanometers wide and up to 40 micrometers long - are grown on a copper substrate using a chemical vapor deposition process. The copper substrate forms droplets that absorb the zinc and oxygen vapors and deposits the ZnO on the substrate. As the nanowire grows, the zinc pushes the droplets up from the surface, but some copper remains inside the nanowire�s crystal lattice. In a new paper, NIST chemists report using a variety of measurement techniques to learn that the ZnO wires contain a surprising amount of copper - between 5 and 15 percent. High-resolution imaging studies of ZnO nanowires reveal that the copper manages to fit into zinc oxide�s regular crystalline structure without disrupting it. �It is in there somewhere,� explains chemist Susie Eustis. Because the copper can be easily detected when you know what to look for, she says, researchers plan to use it to better understand the crystal structure of ZnO nanowires with an eye toward manipulating the nanowires to improve performance. �The copper acts like a smart tag that you put on an animal in the wild to trace where it travels,� says Eustis. The role copper plays in ZnO nanowires is ambiguous. Published studies differ on whether the copper increases or decreases the nanowires� photoluminescence. Eustis and colleagues found that the copper in the nanowire increases the output of visible light but at the expense of ultraviolet emission. In addition to determining the role copper plays in ZnO nanowires, the researchers plan to learn how to grow uniform nanowires that may one day be used in commercial products. This research is part of ongoing studies to find the best methods to determine the concentration and distribution of atoms inside nanostructures. |
|
|
|
|
Related topics - search form: |
|
|