|
Related Topics: |
|
Current Research Articles: |
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Gold can be magnetic on the nanoscale |
|
Atlanta - Physicists at the Georgia Institute of Technology have made two important findings regarding gold on the nanoscale. They found that applying an electrical field on a surface-supported gold nanocluster changes its structure from a three-dimensional one to a planar flat structure. In another paper, they relate their discovery that gold in this size regime can be made magnetic through oxygenation of gold nanowires. They also found that up to a certain length, oxygenated gold nanowires behave as a conducting metal, but beyond that, they become insulators. This marks the first time on the nanoscale that such a metal-to-insulation transition has been found on the nanoscale. Both findings are important predictions that could some day be implemented as control parameters governing the chemical and physical material properties employed in nanotechnology. |
|
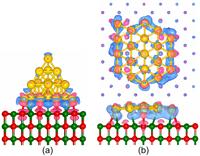
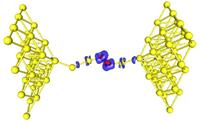
The researchers focused their theoretical investigations on gold nanostructures because of the well known chemical inertness of gold in the bulk form, allowing one to maintain samples with minimal influence on the environment. “However, we again find that small is different,” said Uzi Landman, Regents’ and Institute Professor, holder of the F.E. Callaway Chair, and director of the Center for Computational Materials Science, repeating a phrase that he coined and has used often for close to two decades. “On the nanoscale, even gold becomes a potent catalyst, exhibiting new and surprising, chemical, mechanical, electrical and magnetic behavior, which could not have been extrapolated or predicted on the basis of our knowledge about this substance in the bulk form. Some of these systems may find technological uses in nanocatalysis and as chemical and electrical sensors,” Landman added. For the first study, which appears in the February 8 edition of Physical Review Letters, Landman and Research Scientist Bokwon Yoon performed first-principles quantum mechanical computer simulations of a 20-atom gold nanocluster adsorbed on the surface of a film of magnesium oxide (MgO), an insulator which itself is supported by an underlying metallic silver substrate. The optimal configuration of the adsorbed gold nanocluster depends on the thickness of the underlying MgO film, and for an eight-layer film it was found to be a three-dimensional four-sided pyramid, with one of the sides contacting the magnesium oxide surface. However, the researchers discovered that under the influence of an externally applied electric field, the aforementioned three-dimensional shape of the gold nanocluster is no longer the energetically favored structure. Instead, the optimal structure becomes a flat planar 20-atom gold island spread on the MgO surface. Turning off the applied field or reversing it’s direction results in reverting the structure back to the pyramidal one. The origin of the nanocluster morphological change was found to relate to accumulation of excess electronic charge at the interface between the cluster and the magnesium oxide film. This excess charge, which stabilizes the planar nanocluster structure, originates from the underlying silver substrate, and it’s ability to penetrate to the cluster interface through the eight-layer thick MgO film depends on the presence of the externally applied driving electric field. The researchers also discovered that the chemical activity of the adsorbed gold nanocluster varied significantly under the influence of the applied field, enhancing the low-temperature catalytic oxidation of CO to carbon dioxide. “We found that we can change in a controllable manner the physical as well as the chemical properties of the adsorbed nanostructure by applying an external voltage across the supported gold nanocluster,” said Landman. “ I believe that this finding may introduce a potent control parameter into the chemistry of materials. The newly proposed method for tuning and controlling the structure and reactivity of nanostructures through the application of external electric fields may open novel directions and increase the range and applications of nanocatalytic systems and chemically based sensors and catalytic switches.” The second finding, which appears in the February 1 edition of the same journal, answers the question of what happens when a nanowire of gold is pulled in the presence of oxygen. In these studies Landman, Postdoctoral Fellow Chun Zhang and Senior Research Scientist Robert Barnett used first-principles simulations and quantum electrical transport calculations. They found that oxygenated gold nanowires exhibit different properties, depending on whether the oxygen is incorporated in a molecular form or as individual atoms. Indeed, some of these theoretical results offer a new interpretation of recent laboratory experiments on oxygenated gold nanowires. In the case of incorporation of molecular oxygen into the gold nanowire, the simulations revealed that the nanowires can be stretched to a significantly longer extent than pure gold nanowires – in other words, the adsorbed oxygen molecule serves as a reinforcing clamp. Furthermore, the simulations predict that up to a certain stretching distance (typically up to wires that resemble a stretched necklace of about six gold atoms and an embedded oxygen molecule), such nanowires will conduct electrons similar to a pure gold nanowire. These results have been confirmed experimentally. Moreover, the simulations predict that oxygenated gold nanowires extended beyond a length of about six gold atoms become insulating. The conducting state can be restored by a slight contraction of the wire, thus allowing distance - dependent sensitive metal-to-insulator nano-switching. When individual oxygen atoms, rather than an oxygen molecule, are incorporated in the gold nanowire, the elongation range was found to be limited and the electrical conductance was predicted to be lower than in the previous case of molecular oxygen incorporation. However, a surprising finding was made for such wires, predicting the emergence of magnetism with the magnetic moments localized on the embedded oxygens and on the neighboring gold nanowire atoms. “It’s a very exotic thing,” said Landman of the phenomenon. “Finding materials that have magnetic properties when their bulk form doesn’t have those properties is very interesting from a fundamental point of view, and may have certain future technological applications.” The Georgia Institute of Technology is one of the nation's premiere research universities. Ranked seventh among U.S. News & World Report's top public universities, Georgia Tech's more than 18,000 students are enrolled in its Colleges of Architecture, Computing, Engineering, Liberal Arts, Management and Sciences. Tech is among the nation's top producers of women and African-American engineers. The Institute offers research opportunities to both undergraduate and graduate students and is home to more than 100 interdisciplinary units plus the Georgia Tech Research Institute. |
|
|
|
|
Related topics - search form: |
|
|