|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Polymers 'battered' with nanoparticles could create self healing paints and clever packaging |
|
Research chemists at the University of Warwick have devised an elegant process which simply and cheaply covers small particles of polymer with a layer of silica-based nanoparticles. The final result provides a highly versatile material that can be used to create a range of high performance materials such as; self healing paints, and clever packaging that can be tailored to let precise levels of water, air or both pass in a particular direction. |
|
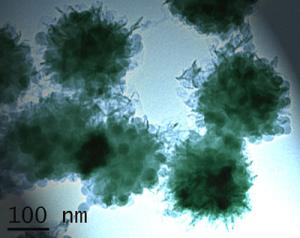
The research, led by Dr Stefan Bon of University of Warwick's Department of Chemistry, has created a "soap free emulsion polymerization process" which makes colloid particles of polymer dispersed in water and in one simple step introduces nanometre sized silica based particles to the mix. These silica based nanoparticles (about 25 nanometre in size) then coats the polymer colloids with a layer "battering" it almost like a fish can be battered in bread crumbs. This process creates a very versatile polymer latex product. It can be used to create scratch resistant paints in which the scratches heal themselves. It can be fine tuned to produce polymer based packaging which will allow water or air to pass through the packaging in tailored ways. The resultant rough textured spherical shapes also lend themselves to the creation of sheets with polymer that present much more surface area than usual allowing more efficient interaction with other materials. The versatility of this process did not stop there. By exposing the material to a second simple step which deposited another polymer layer on top of the already silica based nanoparticles "battered" polymers the researchers were able to produce particles with an even greater range of properties and uses. The image shows such a multi-layered polymer colloid and was taken with a transmission electron microscope. Click on the picture to be taken to a larger hi res version. Industrialists will be interested not just in the versatility of the end product but the ease and cost effectiveness of the process. The Warwick research team has worked on a number of other processes that coated polymers in forms of protection but they all required a number of steps to produce the end result. This new process cuts dramatically the time needed to create such materials and its single step can already be produced on a mass scale with currently used industrial equipment. The amount of material that one can harvest from the process will also impress industrialists as the Warwick team showed that the useful product can easily be made up to around 45% of the volume of each water-based solution used in their process. This compares with figures of as of little as 1 to 10 % for comparable multi-step processes that make these complex particles. |
|
|
|