|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Collapse of helium’s chemical nobility predicted by Polish chemist |
|
140 years since its discovery, and despite the best endeavours of many scientists, helium, the lightest of the 'noble' gases, still stubbornly refuses to enter into any chemical alliance. Now a new glimmer of hope has emerged from poland as a chemist at the university of warsaw has calculated that two new compounds containing a helium-oxygen bond could be formed. |
|
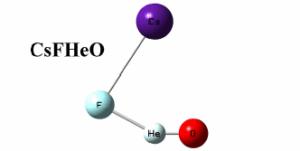
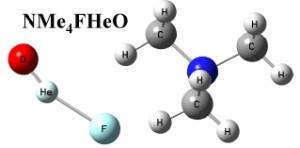
Considered to be the smallest, most chemically inert and least polarizable of the 117 known chemical elements, helium has been challenging chemists for generations. Unfortunately, no-one has managed to confirm experimentally the existence of either HeBeO [considered an example of ‘weak interactions’ rather than a genuine chemical bond (Frenking et al. 1986)] or HHeF [with a lifetime in the femto–picosecond range (Wong 2000 and Gerber et al. 2001)], two important species previously predicted, nor a number of others. The two new molecular species to bind helium to oxygen, predicted using theory, CsFHeO and NMe4FHeO are derivatives of a metastable [F- HeO] anion first theorized in 2005 by a group from taiwan led by Prof. Hu. The scientist responsible for performing these new quantum chemical calculations is Dr Wojciech Grochala from ICM and the faculty of chemistry, the University of Warsaw. Speaking of his results, Dr Grochala said, "The molecules are not as peculiar as they might appear at first light; the idea is to preserve the metastable character of the fragile [F– HeO] entity by attaching it to a weakly coordinating cation (such as Cs+ or NMe4+) to achieve electric neutrality. the resulting species exhibit a He–O bond with an electronic dissociation energy on the singlet potential energy surface (PES) as large as half an eV for the tetramethylammonium derivative." Unfortunately, the kinetic stability of the molecules in question is limited by a crossing of the singlet–triplet PESs and additionally by facile decomposition along the bending channel, both factors considerably limiting their lifetime. The implication for a real world search for these molecules is that they should besought at a temperature of a few kelvin at most. Commenting on how this could be achieved, Dr Grochala said, "The synthesis of both species might begin with the unusual hypofluorites, CsOF and NMe4 of, embedded in an ultracold helium droplet. Laser excitation of the O-F chemical bond should allow for insertion of a helium atom into the bond and for spectroscopic observation of the short– living molecules. Of course, such experiments are quite challenging but this is what makes modern chemistry so much fun". He added, "Despite various difficulties I am really excited about the predictions". |
|
|
|