|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Purer water made possible by Sandia advance |
|
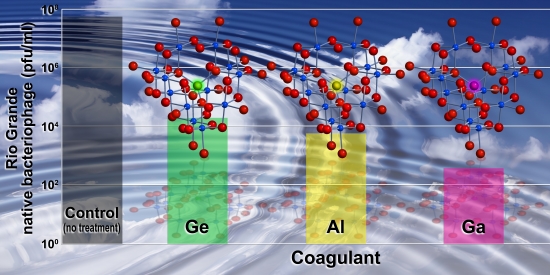
ALBUQUERQUE, N.M. - By substituting a single atom in a molecule widely used to purify water, researchers at Sandia National Laboratories have created a far more effective decontaminant with a shelf life superior to products currently on the market. Sandia has applied for a patent on the material, which removes bacterial, viral and other organic and inorganic contaminants from river water destined for human consumption, and from wastewater treatment plants prior to returning water to the environment. “Human consumption of ‘challenged’ water is increasing worldwide as preferred supplies become more scarce,” said Sandia principal investigator May Nyman. “Technological advances like this may help solve problems faced by water treatment facilities in both developed and developing countries.” |
|
|
The study was published in June 2009 in the journal Environmental Science & Technology (a publication of the American Chemical Society) and highlighted in the June 22 edition of Chemical & Engineering News. Sandia is working with a major producer of water treatment chemicals to explore the commercial potential of the compound. The water-treatment reagent, known as a coagulant, is made by substituting an atom of gallium in the center of an aluminum oxide cluster - itself a commonly used coagulant in water purification, says Nyman.
The substitution isn’t performed atom by atom using nanoscopic tweezers but rather uses a simple chemical process of dissolving aluminum salts in water, gallium salts into a sodium hydroxide solution and then slowly adding the sodium hydroxide solution to the aluminum solution while heating. “The substitution of a single gallium atom in that compound makes a big difference,” said Nyman. “It greatly improves the stability and effectiveness of the reagent. We’ve done side-by-side tests with a variety of commercially available products. For almost every case, ours performs best under a wide range of conditions.” Wide-ranging conditions are inevitable, she said, when dealing with a natural water source such as a river. “You get seasonal and even daily fluctuations in pH, temperature, turbidity and water chemistry. And a river in central New Mexico has very different conditions than say, a river in Ohio.” The Sandia coagulant attracts and binds contaminants so well because it maintains its electrostatic charge more reliably than conventional coagulants made without gallium, itself a harmless addition. The new material also resists converting to larger, less-reactive aggregates before it is used. This means it maintains a longer shelf life, avoiding the problem faced by related commercially available products that aggregate over time. “The chemical substitution [of a gallium atom for an aluminum atom] has been studied by Sandia’s collaborators at the University of California at Davis, but nobody has ever put this knowledge to use in an application such as removing water contaminants like microorganisms,” said Nyman. The project was conceived and all water treatment studies were performed at Sandia, said Nyman, who worked with Sandia microbiologist Tom Stewart. Transmission electron microscope images of bacteriophages binding to the altered material were achieved at the University of New Mexico. Mass spectroscopy of the alumina clusters in solution was performed at UC Davis. The work was sponsored by Sandia’s Laboratory Directed Research Development office. |
|
|
|