|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Enzymes |
|
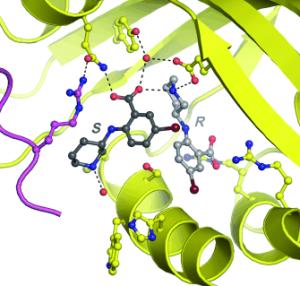
In the binding pockets of enzymes their natural binding partners fit exactly. The principle by which many pharmacological agents work also relies on the fact that these substances fit exactly into the pockets of specific enzymes. Not only the chemical properties but also the shape of the pocket determines if a molecule fits or not. Just as a left glove doesn’t fit onto a right hand, only one of the mirror images normally fits into the binding site of the enzyme. Molecules that are mirror images are called enantiomers. Rolf Breinbauer, Wulf Blankenfeldt, and Matthias Mentel at the Max Planck Institute for Molecular Physiology in Dortmund, the University of Leipzig, and the Technical University of Graz (Austria) report a previously unknown binding variation in the journal Angewandte Chemie: for the first time, they have discovered a drug whose two enantiomers can both dock in the pocket of an enzyme at the same time. |
|
In most cases, only one enantiomer of a pharmacon is effective; the other is simply unnecessary ballast. Occasionally, the mirror-image molecule can even inhibit the activity of the pharmacon, produce counterproductive effects, or cause other undesired side effects. In isolated cases, these can turn out to be dangerous, as was made painfully evident by the thalidomide scandal: Whereas one enantiomer of thalidomide is a well-tolerated, effective sleep-inducing and sedative drug, its mirror image led to severe deformations in the unborn children of pregnant patients. Consequently, legal regulations today require that only enantiomerically pure medications be brought on the market. In the screening used in pharmaceutical research, mixtures of the two mirror images are first tested together. Subsequently, the binding properties of both forms with the target protein are examined to see which of the two forms is the active one. These experiments have led to the understanding that only a single enantiomer fits into the binding site. In rare cases, it has also been observed that both enantiomers can individually bind to the enzyme, but never at the same time. The scientists were surprised to discover entirely different behavior from enantiomers: Both of the enantiomers of an inhibitor being tested were bound simultaneously in the pocket of the enzyme. The discovery may open up interesting new possibilities in pharmaceutical research, for example in fragment-based approaches. In this technique, small bioactive molecular fragments are initially sought for use in combination with other fragments to construct effective pharmaceuticals. |
|
|
|