|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Lithium and Beryllium No Longer "Lack Chemistry" |
|
Even though the lightest known metals in the universe, lithium (Li) and beryllium (Be), do not bind to one another under normal atmospheric or ambient pressure, an interdisciplinary team of Cornell scientists predicted in the Jan. 24 issue of Nature that Li and Be will bond under higher levels of pressure and form stable Li-Be alloys that may be capable of superconductivity. Superconductivity is the flow of electricity with zero resistance. The Inorganic, Bioinorganic and Organometallic Chemistry program at the National Science Foundation (NSF) supported the research because little work had been done to predict the properties of metals under high pressure. |
|
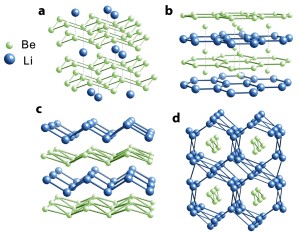
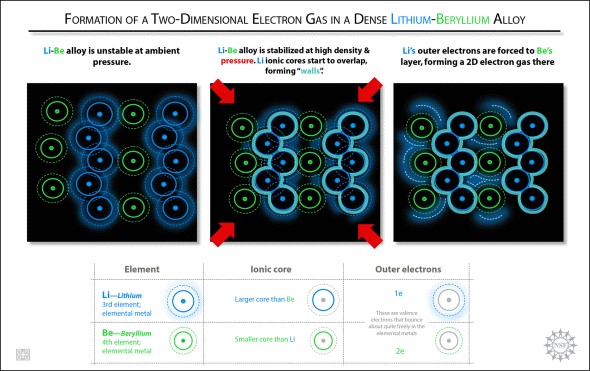
"We found that chemists working on inorganic compounds and inorganic reactions under high pressure were interested in the predictions and felt it would stimulate useful interactions between theorists and experimentalists," said NSF Program Manager Michael Clarke. Of the four stable Li-Be alloys predicted by the scientists' computational study, the alloy with the ratio of one Li atom to one Be atom (LiBe) shows the greatest potential for superconducting applications. A most unexpected finding in the study is the predicted existence of two-dimensional electron gas layers within a tightly compressed three-dimensional LiBe compound. "It's like taking a nice layer cake, squeezing the hell out of it, and lo and behold, out of what would be expected to be a jumbled-up mess, there emerges a neat hazelnut cream layer," said co-author Roald Hoffmann, the 1981 chemistry Nobel laureate and Cornell's Frank H.T. Rhodes Professor in Humane Letters Emeritus. But it makes sense, according to co-author Neil Ashcroft, Cornell's Horace White Professor of Physics Emeritus. When layers of Li and Be are squeezed together at elevated pressures ranging from five to 10 times greater than the pressure at which diamond forms, outer electrons from the Li layer get squeezed into the vicinity of the Be layer, forming two-dimensional gas layers. "It is extraordinary that such remarkably two-dimensional behavior emerges from the conjunction of two such �simple' constituents. It is actually a fine example of �emergent' phenomena," Ashcroft said. He added that they do not yet know whether their theoretical Li-Be alloys will become notable superconductors but creating and testing the compounds would be relatively simple.
Ji Feng, now a postdoctoral researcher at Harvard, is lead author of the Nature paper. Richard Hennig, a Cornell assistant professor in materials science and engineering, is an additional co-author of the paper. The research was supported by NSF Division of Chemistry grant #0613306; Division of Materials Research grant #0601461; and Division of Earth Sciences grant #0703226. |
|
|
|
|
Related topics - search form: |
|
|