|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
A molecular ripcord for chemical reactions |
|
This allowed them to initiate chemical reactions with mechanical force. This discovery paves the way to developing materials capable of repairing themselves under the influence of mechanical tension. The results of their research were published online on 6 April 2009 in the new international journal Nature Chemistry.
|
|
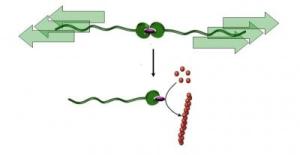
Molecular ripcord The research team (Dr. Alessio Piermattei, Dr. Karthik Sivasubramanian and Dr. Rint Sijbesma) of the Institute for Complex Molecular Systems (ICMS) and the Department of Chemical Engineering and Chemistry, both at TU/e, is the first to have demonstrated that a catalyst can be switched from a dormant to an active state (see illustration) by pulling on a polymer chain, a "molecular ripcord." The researchers were able to use this catalyst to initiate a variety of chemical reactions, including polymerizations (formation of polymer chains from small molecular building blocks called monomers).
Self-repairing materials This discovery paves the way to creating self-repairing materials that strengthen under the influence of mechanical stress. If a material were to tear, for example, this would simultaneously break the metal complex in half, thereby activating the catalyst, and the material would be instantly repaired. This work will also lead to research into other applications in which it should be possible to turn chemical reactions on and off as desired. Potential applications include the injection molding of plastic objects, where the technique could be used to simplify processing, or microscale chemical synthesis.
How does it work; weakest link The researchers packed a catalytically active metal ion completely in using two molecular caps (ligands). They attached two polymer chains to these caps, creating a long chain with a metal complex in the center. These complexes were dissolved in a liquid that was irradiated with ultrasound, causing bubbles to form in the liquid. When these bubbles imploded, they created an extremely strong current that stretched the chains and ultimately broke its weakest link – the metal complex – in two. The cap on one end was now broken off from the active metal ion, which allowed the metal ion to become catalytically active. In other words, it could now accelerate chemical reactions. This research was sponsored with an ECHO project subsidy from NWO (Netherlands Organization for Scientific Research). The subsidy, in the amount of 240,000 euros, is intended to promote outstanding chemical research, especially on creative and risky ideas.
Abstract of the article: Homogeneously catalysed reactions can be 'switched on' by activating latent catalysts. Usually, activation is brought about by heat or an external chemical agent. However, activation of homogeneous catalysts with a mechanical trigger has not been demonstrated. Here, we introduce a general method to activate latent catalysts by mechanically breaking bonds between a metal and one of its ligands. We have found that silver(i) complexes of polymer-functionalized N-heterocyclic carbenes, which are latent organocatalysts, catalyse a transesterification reaction when exposed to ultrasound in solution. Furthermore, ultrasonic activation of a ruthenium biscarbene complex with appended polymer chains results in catalysis of olefin metathesis reactions. In each case, the catalytic activity results from ligand dissociation, brought about by transfer of mechanical forces from the polymeric substituents to the coordination bond. Mechanochemical catalyst activation has potential applications in transduction and amplification of mechanical signals, and mechanically initiated polymerizations hold promise as a novel repair mechanism in self-healing materials. |
|
|
|