|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Tetrahedra Packing Record |
|
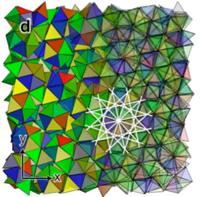
Two Kent State University professors are part of a team of researchers who recently uncovered a way to pack tetrahedra, considered to be the simplest shaped regular solids with its four triangular sides, more densely than ever before. Peter Palffy-Muhoray, professor of chemical physics and associate director of the Liquid Crystal Institute at Kent State, and Xiaoyu Zheng, assistant professor in Kent State’s Department of Mathematical Sciences, along with four colleagues at the University of Michigan and one at Case Western Reserve University, have broken a world record for packing the most tetrahedra into a given volume. Their findings were featured in the Dec. 10, 2009 issue of Nature, one of the leading international scientific journals, in an article co-authored by the seven researchers. The article is titled “Disordered, quasicrystalline and crystalline phases of densely packed tetrahedra.” |
|
The researchers were able to obtain the highest packing fraction of 85.03, meaning tetrahedra fill 85.03 percent of the volume of the container. This shattered the previous record of 78.2 percent set by two Princeton University researchers in August 2009. “The question of how best to pack shapes into a volume is an age-old question,” Palffy-Muhoray said. “Johannes Kepler asked how to pack spheres in the early 1600s, and it was only recently proven in 2005 that the best way is to stack them like cannonballs. It is easy to understand how cubes can entirely fill space with no voids, but the packing problem is still unsolved for the simple tetrahedron. Though it’s a simple object, it can’t fill space like cubes, so we wondered how hard tetrahedra would pack when you squeezed them together.” In the process, the Kent State professors and their colleagues discovered that quasicrystals formed when the tetrahedra were compressed. “A crystal is a material structure which repeats periodically,” Palffy-Muhoray explained. “A quasicrystal is similar, but it doesn’t repeat itself exactly, despite its regularity. It’s something quite new, having been discovered only 25 years ago. Not only did we show that tetrahedra can pack much denser than previously thought, but we also found the most remarkable result: that they form quasicrystals. It’s amazing that the simplest solid – the tetrahedron – forms these intricately complicated, amazingly complex structures. This is the first example of particles forming quasicrystals with no interactions other than hard objects bumping into one another. Entropy, which is often associated with disorder and chaos, can in fact create order.” Sharon Glotzer, a professor in the University of Michigan’s departments of Chemical Engineering and Materials Science and Engineering who conceived and designed the study together with Palffy-Muhoray, said, “This is the most complex structure we’ve ever seen arising from purely entropic interactions.” Previous approaches by other researchers began with the geometric constructions and compressing these. Glotzer, Palffy-Muhoray and collaborators started from a random initial structure and compressed it to allow natural evolution toward high-density states. Work began in 2006 with physical experiments being conducted at Kent State involving Palffy-Muhoray and Zheng. “We carried out experiments here at Kent State using tetrahedral dice to see how densely we could pack them,” Zheng said. “We also constructed various motifs. Then, the key computer simulations were carried out at the University of Michigan. By using a new, ingenious scheme, we were able to achieve 85.03 percent packing. When we first started this work, we did not expect the results that we achieved. This whole project has been very exciting.” The results of this research and the formation of quasicrystals offer some interesting and exciting possibilities on how it can be used in real-world applications. “This will enable the production of metamaterials, which are manmade materials that don’t exist in nature, with interesting physical and optical properties,” Palffy-Muhoray said. “Applications are far-ranging, including high-resolution imaging useful for microscopy in medicine and materials science. This new packing method could enable the production of new kinds of materials, useful for computer chips, building materials and fabrics.” Palffy-Muhoray, whose distinguished career includes being elected a Fellow of the American Physical Society in 2008, said this research has been very rewarding. “In trying to understand simple things, you occasionally stumble upon beautiful, complex phenomena,” he said. “This does stand out as one of the most beautiful things we’ve discovered. It’s very satisfying that this simple inquiry would lead to such remarkable results.” Funding support for this research was provided by an Air Force MURI (Multidisciplinary University Research Initiative) grant to Kent State University and by the National Science Foundation. University of Michigan: Entropy alone creates complex crystals from simple shapes, study showsANN ARBOR, Mich.- In a study that elevates the role of entropy in creating order, research led by the University of Michigan shows that certain pyramid shapes can spontaneously organize into complex quasicrystals. A quasicrystal is a solid whose components exhibit long-range order, but without a single pattern or a unit cell that repeats. A paper on the findings appeared in the Dec. 10, 2009 issue of Nature. Researchers from Case Western Reserve University and Kent State University collaborated on the study. Entropy is a measure of the number of ways the components of a system can be arranged. While often linked to disorder, entropy can also cause objects to order. The pyramid shape central to this research is the tetrahedron - a three-dimensional, four-faced, triangular polyhedron that turns up in nanotechnology and biology. "Tetrahedrons are the simplest regular solids, while quasicrystals are among the most complex and beautiful structures in nature. It's astonishing and totally unexpected that entropy alone can produce this level of complexity," said Sharon Glotzer, a professor in the University of Michigan departments of Chemical Engineering and Materials Science and Engineering and principal investigator on the project. The finding may lead to the development of a variety of new materials that derive properties from their structure, said Rolfe Petschek, a physics professor at Case Western Reserve who helped with the mathematical characterization of the structure. "A quasicrystal will have different properties than a crystal or ordinary solid," Petschek said. The scientists used computer simulation to find the arrangement of tetrahedrons that would yield the densest packing - that would fit the most tetrahedrons in a box. The tetrahedron was for decades conjectured to be the only solid that packs less densely than spheres, until just last year when U-M mathematics graduate student Elizabeth Chen found an arrangement that proved that speculation wrong. This latest study bests Chen's organization and discovered what is believed to be the densest achievable packing of tetrahedrons. But Glotzer says the more significant finding is that the tetrahedrons can unexpectedly organize into intricate quasicrystals at a point in the computer simulation when they take up roughly half the space in the theoretical box. In this computer experiment, many thousands of tetrahedrons organized into dodecagonal, or 12-fold, quasicrystals made of parallel stacks of rings around pentagonal dipyramids. A pentagonal dipyramid contains five tetrahedrons arranged into a disk. The researchers discovered that this motif plays a key role in the overall packing. This is the first result showing such a complicated self-arrangement of hard particles without help from attractive interactions such as chemical bonds, Glotzer said. "Our results go to the very heart of phase transitions and to the question of how complex order arises in nature and in the materials we make," Glotzer said. "We knew that entropy on its own could produce order, but we didn't expect it to produce such intricate order. What else might be possible just due to entropy?" Other approaches to solving the tetrahedron packing problem have not involved computer simulations. Researchers instead tried out different arrangements to arrive at the densest structure. That was the approach taken by Chen, who achieved a packing fraction of more than 77 percent, which means the shapes took up more than 77 percent of the space in the box. (Cubes have a 100 percent packing fraction in a cubic box, while spheres pack at only 74 percent.) Rather than "posit what they might do," this computer simulation allowed the tetrahedrons to figure out the best packing on their own according to the laws of statistical mechanics and thermodynamics, said Michael Engel, a postdoctoral researcher at U-M and co-first author of the paper with U-M chemical engineering graduate student Amir Haji-Akbari. In the simulation, the tetrahedrons organized into a quasicrystal and settled on a packing that, when compressed further, used up 83 percent of the space. Engel then reorganized the shapes into a "quasicrystalline approximate," which is a periodic crystal closely resembling the quasicrystal. He found an arrangement that filled more than 85 percent of the space. The researchers are excited about the possible applications of the new structure. "Made of the right materials, this unexpected new tetrahedron quasicrystal may possess unique optical properties that could be very interesting and useful," said Peter Palffy-Muhoray, a professor in the Liquid Crystal Institute at Kent State University and a collaborator on the work. Possible uses include communication and stealth technologies. |
|
|
|