|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Fox Chase Researchers Give Mutants Another Chance |
|
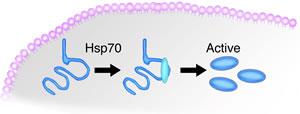
PHILADELPHIA – Researchers at Fox Chase Cancer Center have demonstrated that it might be possible to treat genetic diseases, including some forms of cancer, by "rescuing" the misshapen, useless proteins produced by some mutant genes. In the Feb 2009 issue of the Journal of Biological Chemistry, available online, researchers detail how they were able to restore the function of a mutant human gene in a yeast model of disease by manipulating the available amounts of a so-called chaperone protein, named Hsp70, which helps amino acid chains fold into their proper protein form. |
|
"In some cases, despite a mutation, it is possible to coax a misfolded protein back into a functional conformation," says the paper's lead author Warren Kruger, PhD, senior faculty member at Fox Chase. "In essence, we're using Hsp70 to call a biochemical mulligan, a do-over. "Hsp70 pulls a misfolded amino acid chain apart like a twisted rubber band and allows it to snap back into place, which we found can restore a significant percentage of proteins to working shape," Kruger says. "If this can be done in humans, it could represent a way of reducing the severity – or perhaps correcting – certain hereditary diseases, even some familial cancers." Kruger and his colleagues are currently studying how to adapt these findings to human disease. While the study was done in a yeast model for human disease, humans possess at least nine members of the Hsp70 family of chaperones. Evidence suggests that it might be feasible to modify the chaperone environment in order to give Hsp70 better opportunities to rescue broken proteins. "The more chances we give Hsp70 proteins to try to 'fix' the output of mutant genes, the more chances are that they will succeed," Kruger says. Very often, genetic mutations occur when errors in the DNA code substitute one amino acid for another. It is this sort of mutation that is at the center of a disease called cystathionine ß-synthase (CBS) deficiency, an inherited metabolic disorder that the Kruger laboratory uses as a genetic model. Through experiments performed by Laishram Singh, a post-doctoral fellow in the lab, Kruger learned that exposure to alcohol restored the function of a mutant human CBS protein expressed in genetically engineered yeast cells. This exposure activates heat shock proteins, like Hsp70, which help the cell respond to the shock, or damage, from stress. The sudden excess of Hsp70, in turn, had the side effect of rescuing mutated proteins in addition to those damaged by alcohol. According to Kruger, Hsp70 works in equilibrium with another heat shock protein, Hsp26, as a sort of biochemical damage control system. While Hsp70 acts like a chaperone to restore the function of such proteins, Hsp26 targets the misfolded proteins to the proteosome, the "garbage disposal" of the cell. The two proteins, it seems, function in opposition, competing to clean up damaged proteins within cells. The relationship between these two proteins may provide an opportunity for intervention, Kruger reasons. When Kruger and his colleagues removed the gene encoding Hsp26 from yeast, they saw a nine-fold increase in the amount of properly functioning human CBS protein. "If we can target Hsp26 and take it out of circulation, we might be able to give Hsp70 more room to operate," Kruger says. Funding for this research comes from grants from the National Institutes of Health and the Commonwealth of Pennsylvania. Fox Chase Cancer Center is one of the leading freestanding cancer research and treatments centers in the United States. Founded in 1904 in Philadelphia as the nation's first cancer hospital, Fox Chase became one of the first institutions to be designated a National Cancer Institute Comprehensive Cancer Center in 1974. Today, Fox Chase conducts a broad array of nationally competitive basic, translational, and clinical research, with special programs in cancer prevention, detection, treatment, and community outreach. |
|
|
|