Related Topics: |
|
|
Current News |
Chemistry A to Z |
About Internetchemistry |
Deceiving Cell Walls |
|
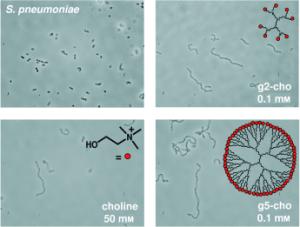
Approximately 1.6 million people die worldwide every year as a result of pneumococcal infection, which causes grave illnesses, including pneumonia, meningitis, and middle-ear infections. Children and the elderly are especially at risk. Vaccines are only effective against a few of the pneumococcal types and increasing resistance to antibiotics is making treatment more difficult. Researchers led by Jesús M. Sanz at the Miguel Hernandez University (Elche, Spain) and Maarten Merkx at the Eindhoven University of Technology (Netherlands) have now introduced a highly promising new approach for the development of drugs to treat pneumococci. As they report in the journal Angewandte Chemie, the scientists copied the choline architecture of the pneumococcal cell wall. They were thus able to trap the choline-binding proteins that have a critical effect on the infectiousness of pneumococcal bacteria. |
|
The cell walls of pneumococci contain special polymers, called teichoic acids, that are equipped with phosphocholine groups and induce a characteristic choline architecture on the cell walls. The choline groups act as docking stations for a number of special proteins that are involved in important processes such as cell-wall division, the release of bacterial toxins, and adhesion to infected tissues. These choline-binding proteins (CBP) contain domains with multiple neighboring choline-binding sites. The protein LytA, for example, has a domain with four choline-binding sites. If choline is added to a culture of pneumococci, the molecules occupy the choline binding sites of the CBPs so that the proteins can no longer bind to the cell walls of the pneumococci. Although the bacteria continue to multiply, the individual cells can no longer separate from each other, which results in long chains of linked cells. In addition, the toxin-releasing self-destruction (autolysis) typical of pneumococci at the end of their life cycle is stopped. However, choline is not suitable for use as a drug because an effective dose would be far too high. The researchers thus developed the foundation for a new drug that binds CBP much more strongly than individual choline molecules. Their trick: the drug imitates the choline architecture of the cell wall by presenting multiple choline groups. As a scaffold for their assembly of choline groups, the researchers chose to use dendrimers (tree-like branched molecules), attaching the choline groups to the tips of the “branches”. The choline ends can simultaneously occupy multiple choline binding sites of the CBP. The dendrimer frameworks are flexible enough to meet the spatial demands of the application. The required dosage of this CBP inhibitor lies within the range that is acceptable for pharmaceuticals. |
|
|
|