|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Carbon Dioxide Capture |
|
Until recently, factory smokestacks that produced nothing but carbon dioxide and water vapor were considered exemplary. Now CO2 has become notorious as a greenhouse gas, and the danger of climate change has become one of the most pressing environmental problems of our time. How can we slow the increasing release of CO2? Efficient methods for the separation of this greenhouse gas from industrial exhaust are being sought. Korean researchers have now developed a porous material that can bind and store CO2 efficiently and highly selectively. As Myunghyun Paik Suh and Hye-Sun Choi report in the journal Angewandte Chemie (1), the lattice-like network contains flexible “columns” that can open the pores of the three-dimensional lattice for CO2. |
|
|
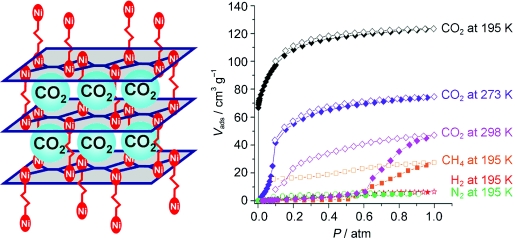
Many porous materials are able to absorb CO2 and other gas molecules. However, the selective, room-temperature extraction of CO2 at atmospheric pressure from industrial exhaust containing other gases such as nitrogen, methane, and water remains a major technical challenge. The research team has now developed porous, three-dimensional networks of coordination polymers. Various nickel complexes and organic molecules are used as building blocks that assemble into two-dimensional lattice-like planes, which in turn grow into stacks held together by “columns”. The special trick in this case is that the columns are not rigid, but very flexible. The corresponding cavities in the structure are thus of variable size and can adjust to the guest molecules that enter.
The symmetric molecule carbon dioxide has a permanent electrical quadrupole moment that can be described as two electrical dipoles sitting back-to-back and pointing in opposite directions. This quadrupole interacts with the three-dimensional lattice, and this effect causes the columns to open the “gates”, allowing the gas to enter the cavities. In contrast, nitrogen, hydrogen, and methane have much smaller quadrupole moments. The pores thus remain closed to them. The exclusion of nitrogen, which makes up a large proportion of air, is essential for any potential CO2 capture. In addition, the new nickel-containing materials are stable at temperatures up to 300 °C and are air- and water- stable - also an important requirement for potential industrial application. If the surrounding pressure is reduced, the stored CO2 is released. This type of material is thus suited for processes in which carbon dioxide must be cyclically stored and then released through a change in pressure. |
|
|
|