|
|
|
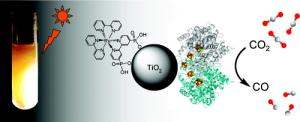
A hybrid enzyme-nanoparticle system is described for achieving clean reduction of CO2 to CO using visible light as the energy source. An aqueous dispersion of TiO2 nanoparticles modified by attachment of carbon monoxide dehydrogenase (CODH) and a Ru photosensitizer produces CO at a rate of 250 µmol of CO (g of TiO2)-1 h-1 when illuminated with visible light at pH 6 and 20 °C.
[Credit: JACS, DOI 10.1021/ja910091z]
|
The results are reported in the online edition of the Journal of the American Chemical Society. Not only is it a demonstration that an abundant compound can be converted into a commercially useful compound with considerably less energy input than current methods, it also is a method not so different from what organisms regularly do. “This is a first step in showing it’s possible, and imagine microbes doing something similar,” Ragsdale said. “I don’t know of any organism that uses light energy to activate carbon dioxide and reduce it to carbon monoxide, but I can imagine either finding an organism that can do it, or genetically engineering one to channel light energy to coax it to do that.” In this collaboration between Ann Arbor and Oxford, Ragsdale’s laboratory at the U-M Medical School does the biochemistry and microbiology experiments and Armstrong’s lab performs the physical- and photochemical applications. Ragsdale and his associates succeeded in using an enzyme-modified titanium oxide to get carbon dioxide’s electrons excited and willing to jump to the enzyme, which then catalyzes the reduction of carbon dioxide to carbon monoxide. A photosensitizer that binds to the titanium allows the use of visible light for the process. The enzyme is more robust than other catalysts, willing to facilitate the conversion again and again. The trick: It can’t come near oxygen. “By using this enzyme, you put it into a solution that contains titanium dioxide in the presence of a photosensitizer,” he said. “We looked for a way that seems like nature’s way of doing it, which is more efficient.” Armstrong notes that “essentially it shows what is possible were we to be able to mass-produce a catalyst with such properties”. The direct product - carbon monoxide - is a desirable chemical that can be used in other processes to produce electricity or hydrogen. Carbon monoxide also has significant fuel value and readily can be converted by known catalysts into hydrocarbons or into methanol for use as a liquid fuel. Although carbon monoxide serves as a source of energy and biomass for microbes, it is toxic for animals and this risk needs to be managed when it is generated or used in chemical reactions. Research in Ragsdale’s lab was funded by the National Institute of General Medical Sciences at the National Institutes of Health. Ragsdale, a professor of biological chemistry at the U-M Medical School, is a fellow of the Michigan Memorial Phoenix Energy Institute, which develops, coordinates and promotes multidisciplinary energy research and education at U-M.
|